HDAC inhibitor & other new products of Cellagen Tech.
본문
HDAC inhibitor & other new products of Cellagen Technology
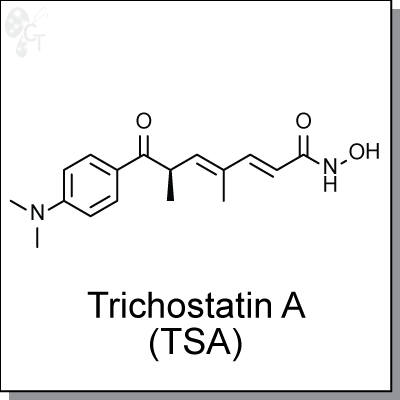
Product Name: Trichostatin A (TSA) | HDAC inhibitor (#C8742)
|
Trichostatin A (TSA) is a potent histone deacetylase (HDAC) inhibitor. It inhibits HDAC 1, 2, 3, 6, 10, 11 at IC50s of less than 10 nM, with over 300-fold selectivity against class IIa HDACs.[1] TSA affects DNA replication and gene expression by inhibiting HDAC activity and therefore altering the histone modifications and access of DNA inside chromatin.
Trichostatin A induces apoptosis and cell growth arrest at both G and G/M phases. As HDACs are overexpressed in many cancer types, TSA is widely used to probe the tumorigenesis mechanism targeting HDAC.[2] Trichostatin A was found to prevent the differentiation of embryonic stem cell,[3] while TSA treatment increased functional characteristics of human ESC/iPSC-derived cardiomyocytes.[4] |
Details
|
Chemical Formula: |
|
C17H22N2O3 |
|
CAS No.: |
|
58880-19-6 |
|
Molecular weight: |
|
302.37 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Brown |
|
Chemical name: |
|
(R,2E,4E)-6-(4-(dimethylamino)benzoyl)-N-hydroxy-4-methylhepta-2,4-dienamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
Trichostatin A, TSA |
|
Storage: |
|
For longer shelf life, store solid powder or DMSO solution at -20oC |
|
References 1. Lobera M, et al. Selective class IIa histone deacetylase inhibition via a nonchelating zinc-binding group. Nat Chem Biol. 2013; 9(5):319-25. Pubmed ID: 23524983 2. Timmermann S, et al. Histone acetylation and disease. Cell Mol Life Sci. 2001; 58(5-6):728-36. Review Pubmed ID: 11437234 3. Lee JH, et al. Histone deacetylase activity is required for embryonic stem cell differentiation. Genesis. 2004; 38(1):32-8. Pubmed ID: 14755802 4. Otsuji TG, et al. Dynamic link between histone H3 acetylation and an increase in the functional characteristics of human ESC/iPSC-derived cardiomyocytes. PLoS One. 2012; 7(9):e45010 Pubmed ID: 11437234 |
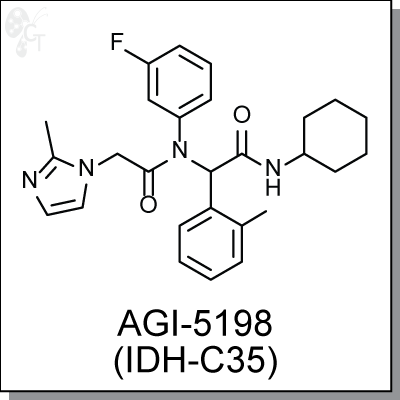
Product Name: AGI-5198 (IDH-C35) | IDH1 inhibitor (#C2519)
|
AGI-5198 (IDH-C35) is a potent and selective isocitrate dehydrogenase 1 (IDH1) inhibitor specifically against R132H/C mutants (mIDH1) with IC50 at 100 nM range. AGI-5198 does not inhibit wild-type IDH1 or any of the examined IDH2 isoforms (IC50 > 100 µM).[1] AGI-5198 inhibits R-2-hydroxyglutarate (R-2HG) production by mIDH1. AGI-5198 also induces demethylation of histone H3K9me3 and expression of genes associated with gliogenic differentiation.
In both in vitro and in vivo studies, AGI-5198 inhibited the growth of glioma cells carrying mutated but not wild type IDH1, yet with no appreciable changes in genome-wide DNA methylation. These data suggest that mIDH1 may promote glioma growth through mechanisms beyond its well-characterized epigenetic effects. AGI-5198 could serve as a very useful chemical tool to probe the mechanism and treatment of mIDH1- carrying tumors. |
Details
|
Chemical Formula: |
|
C27H31FN4O2 |
|
CAS No.: |
|
1355326-35-0 |
|
Molecular weight: |
|
462.56 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
N-cyclohexyl-2-(N-(3-fluorophenyl)-2-(2-methyl-1H-imidazol-1-yl)acetamido)-2- (o-tolyl)acetamide |
|
Solubility: |
|
Up to 25 mM in DMSO |
|
Synonyms: |
|
AGI-5198, AGI5198, IDH-C35 |
|
Storage: |
|
For longer shelf life, store solid powder or DMSO solution at -20oC |
|
Reference 1. Rohle D, et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013; 340(6132):626-30. Pubmed ID: 23558169 |
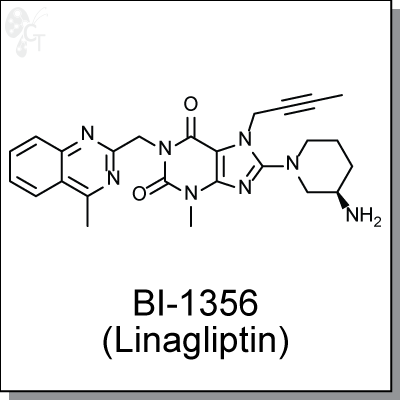
Product Name: BI-1356 (Linagliptin) | DPP-IV/DPP-4 inhibitor (#C2135)
|
BI-1356 (Linagliptin) is a highly potent and selective dipeptidyl peptidase 4 (DPP-4) inhibitor (IC50 = 1 nM) for treatment of type II diabetes. [1] BI-1356 can increase incretin levels (GLP-1 and GIP), which increases insulin secretion and inhibits glucagon release, decreases gastric emptying, and decreases blood glucose levels. BI-1356 shows 10,000-fold more selectivity for DPP-4 against other protease/peptidases, including DPP-8, DPP-9, trypsin, plasmin, and thrombin.[2] |
Details
|
Chemical Formula: |
|
C25H28N8O2 |
|
CAS No.: |
|
668270-12-0 |
|
Molecular weight: |
|
472.54 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
(R)-8-(3-aminopiperidin-1-yl)-7-(but-2-ynyl)-3-methyl-1-((4-methylquinazolin-2-yl) methyl)-1H-purine-2,6(3H,7H)-dione |
|
Solubility: |
|
Up to 25 mM in DMSO |
|
Synonyms: |
|
BI-1356, BI1356, Linagliptin, Tradjenta, Trajenta |
|
Storage: |
|
For longer shelf life, store solid powder or DMSO solution at -20oC |
|
References 1. Eckhardt M, et al. 8-(3-(R)-aminopiperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2- ylmethyl)-3,7-dihydropurine-2,6-dione (BI 1356), a highly potent, selective, long-acting, and orally bioavailable DPP-4 inhibitor for the treatment of type 2 diabetes. J Med Chem. 2007; 50(26):6450-3. Pubmed ID: 18052023 2. Thomas L, et al. (R)-8-(3-amino-piperidin-1-yl)-7-but-2-ynyl-3-methyl-1-(4-methyl-quinazolin-2- ylmethyl)-3,7-dihydro-purine-2,6-dione (BI 1356), a novel xanthine-based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase-4 inhibitors. J Pharmacol Exp Ther. 2008; 325(1):175-82. Pubmed ID: 18223196 |
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
C8742 |
Trichostatin A (TSA) | HDAC inhibitor |
2mg, 10mg & 50mg |
|
C2519 |
AGI-5198 (IDH-C35) | IDH1 inhibitor |
5mg, 25mg & 100mg |
|
C2135 |
BI-1356 (Linagliptin) | DPP-IV/DPP-4 inhibitor |
5mg, 25mg & 100mg |
* 관련제품 정보
Stem Cell Pathway and Chemical Modulators
Stem Cell Pathway Modulating Compounds
▣ 관련 페이지 ; Cellangen Technology
- 이전글Minute™ Yeast Mitochondria Enrichment Kit 14.08.11
- 다음글UCH-L1 inhibitor & other new products of Cellagen Tech. 14.08.11
댓글목록
등록된 댓글이 없습니다.