Red fluorescent protein FusionRed
본문
Red fluorescent protein FusionRed
Supermonomeric red fluorescent protein optimized for fusions and long-term experiments
- Superior performance in fusions
- Low cytotoxicity
- Fast maturation, high pH-stability and photostability
- Proven suitability to generate stably transfected cell lines
- Recommended for protein labeling and long-term experiments
FusionRed is a red fluorescent protein characterized by improved performance in fusions and low toxicity [Shemiakina et
al., 2012]. FusionRed lacks the residual tendency of other monomeric RFPs to dimerize at high concentration and
behaves as a pure monomer at concentrations up to 10 mg/ml in HPLC analysis. Such "supermonomeric" properties
ensure superior efficiency of FusionRed in protein labeling applications, especially in the cells with high expression level.
Similarly to parental mKate2, FusionRed demonstrates fast maturation rate, high pH-stability and photostability, and
significantly lower cytotoxicity than widely used mCherry [Shaner et al., 2004] and mRuby [Kredel et al., 2009].
FusionRed is mainly intended for protein labeling and long-term experiments including generation of transgenic animals.
Main properties
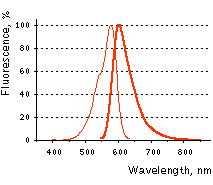
FusionRed normalized excitation (thin line) and emission (thick line) spectra
|
Characteristic |
|
|
Molecular weight, kDa |
26 |
|
Polypeptide length, aa |
232 |
|
Fluorescence color |
red |
|
Excitation maximum, nm |
580 |
|
Emission maximum, nm |
608 |
|
Quantum yield |
0.19 |
|
Extinction coefficient, M-1cm-1 |
95 500 |
|
Brightness* |
18.0 |
|
Brightness, % of EGFP |
53 |
|
pKa |
4.6 |
|
Structure |
supermonomer** |
|
Aggregation |
no |
|
Maturation half-time, min |
130 |
|
Photostability, wide field*** |
150 |
|
Photostability, confocal*** |
176 |
|
Cell toxicity |
not observed |
|
Possible limitations |
superior performance in fusions, low cytotoxicity |
* Brightness is a product of extinction coefficient and quantum yield, divided by 1000.
** Purified recombinant protein behaves as a pure monomer at concentrations of 10 mg/ml as verified by high-
performance liquid chromatography (HPLC)
*** Time to bleach 50% of fluorescent signal brightness.
HPLC analysis of FusionRed in comparison with selected fluorescent proteins loaded in a high concentration.
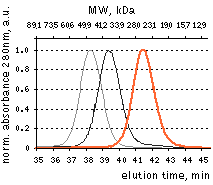
FusionRed – orange line, mKate2 – black line, mNeptune – gray line. Proteins were loaded at a concentration of 10 mg/ml. While HPLC demonstrates reversible dimerization of mKate2 and reveals a dimeric character for the mKate derivative,
far-red fluorescent protein mNeptune [Lin et al., 2009], FusionRed behaves as a pure monomer. Data from Shemiakina
et al., 2012
Recommended filter sets and antibodies
FusionRed can be recognized using Anti-tRFP antibody (Cat.# AB233-AB234) available from Evrogen.
FusionRed can be detected using TRITC filter set or similar. Recommended Omega Optical filter sets are QMAX-Red and
XF102-2.
Performance and use
FusionRed can be easily expressed and detected in a wide range of organisms. Mammalian cells transiently transfected
with FusionRed expression vectors produce bright fluorescence in 10-12 hours after transfection.
Labeling efficiency testing
The performance of FusionRed and mCherry was directly compared in HeLa CCL2 cells for the following targets:
connexin-43, endosomes, vinculin, and the Golgi complex. In this experiment, the cells were transfected with each of
the four target fusions, fixed, and then compared for localization efficiency, which was determined by calculating the
percentage of properly expressing cells versus the total number of transfected cells. FusionRed demonstrated clear
advantage in all four fusions. Data from Shemiakina et al., 2012
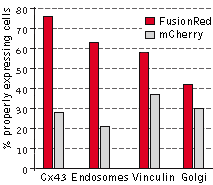
Cytotoxicity testing in HeLa cells
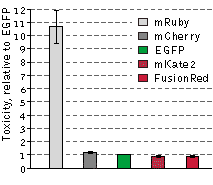
The cytotoxicity of FusionRed relative to selected red fluorescent proteins and EGFP was evaluated in the following
experiment: HeLa cells were transfected with appropriate vectors encoding EGFP or one of the following red fluorescent
proteins: mRuby, FusionRed, mKate2 or mCherry. Next, the EGFP-expressing cells were mixed with those expressing one
of the RFPs, resulting in 4 separate cell mixtures: EGFP and mRuby, EGFP and FusionRed, EGFP and mKate2, and EGFP
and mCherry. 48 hours after transfection, the green-to-red cell ratios were calculated utilizing flow cytometry and each
of the cell mixtures were then plated into 3 plates. After additional 92 hour incubation, the green-to-red cell ratios were
recalculated. Because only living cells were counted for this experiment, the difference between the ratios before and
after the incubation can be assumed to accurately reflect RFP toxicity versus EGFP. mRuby exhibited a more than 10-fold
higher cytotoxicity level compared to EGFP, while the remaining RFPs were almost as cytotoxic as EGFP in this
experiment. Data from Shemiakina et al., 2012
Long-term expression
The excellent suitability of FusionRed for long-term experiments was proved both in experiments with stably transfected cell lines and in transgenic animals. In addition to its low cytotoxicity, FusionRed does not show abnormal lysosomal
localization typical for many fluorescent proteins in long-term expression.
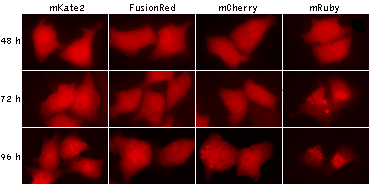
Being expressed in HeLa cells, both mKate2 and FusionRed remain evenly localized in cytoplasm after 96 hours of
cultivation, while mCherry and mRuby show abnormal lysosomal localization. Data from Shemiakina et al., 2012
References:
- Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1989; 264 (36):21522-8. / pmid: 2600080
- Haas J, Park EC, Seed B. Codon usage limitation in the expression of HIV-1 envelope glycoprotein. Curr Biol. 1996; 6 (3):315-24. / pmid: 8805248
- Kredel S, Oswald F, Nienhaus K, Deuschle K, Röcker C, Wolff M, Heilker R, Nienhaus GU, Wiedenmann J. mRuby, a bright monomeric red fluorescent protein for labeling of subcellular structures. PLoS One. 2009; 4 (2):e4391. / pmid: 19194514
- Lin MZ, McKeown MR, Ng HL, Aguilera TA, Shaner NC, Campbell RE, Adams SR, Gross LA, Ma W, Alber T, Tsien RY. Autofluorescent proteins with excitation in the optical window for intravital imaging in mammals. Chem Biol. 2009; 16 (11):1169-79. / pmid: 19942140
- Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987; 48 (5):899-907. / pmid: 3545499
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004; 22 (12):1567-72. / pmid: 15558047
- Shemiakina II, Ermakova GV, Cranfill PJ, Baird MA, Evans RA, Souslova EA, Staroverov DB, Gorokhovatsky AY, Putintseva EV, Gorodnicheva TV, Chepurnykh TV, Strukova L, Lukyanov S, Zaraisky AG, Davidson MW, Chudakov DM, Shcherbo D. A monomeric red fluorescent protein with low cytotoxicity. Nat Commun. 2012; 3 :1204. / pmid: 23149748
Ordering information ;
▣ 관련 페이지 ; Evrogen
- 이전글photoactivatable red fluorescent protein PA-TagRFP 13.04.06
- 다음글Near-infrared fluorescent protein TurboFP650 12.09.24
댓글목록
등록된 댓글이 없습니다.