Epigenetics / Cellagen Technology LLC
본문
Epigenetics / Cellagen Technology LLC
Product Name: BIX-01294 (C2491)
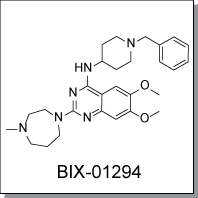
BIX-01294 is a selective inhibitor of G9a histone methyl transferase (G9a HMTase) that impairs G9a HMTase and
generation of H3K9me2 in vitro. In its inhibition of the histone lysine methyltransferases, BIX-01294 does not compete
with cofactor S-adenosyl-methionine. G9a HMTase regulates gene expression including one of the pluripotency genes
Oct4. It is reported that BIX-01294 enhances reprogramming efficiency of neural progenitor cells to the same levels as
when four transcription factors (Oct4, Klf4, Sox2 and c-Myc) were introduced to somatic cells for generation of induced
pluripotent stem cells.
Details
|
Chemical Formula : |
: |
C28H38N6O2 |
|
CAS # : |
|
935693-62-2 |
|
Molecular Weight |
: |
490.64 |
|
Purity : |
>98% | |
|
Formulation : |
|
Pale yellow solid |
|
Solubility : |
|
Soluble in DMSO up to 100 mM |
|
Chemical Name : |
|
2-(Hexahydro-4-methyl-1H-1,4-diazepin-1-yl)-6,7-dimethoxy-N-[1-(phenylmethyl)-4-piperidinyl]-4-quinazolinamine trihydrochloride hydrate |
|
Storage : |
Store solid powder at 4oC desiccated;
Store DMSO solution at -20oC.
|
References
1. Kubicek, S. et al., Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase Mol. Cell.
3rd ed., 25, 473-481, (2007).
2. Shi, Y. et al. A combined chemical and genetic approach for the generation of induced pluripotent stem cells. Cell
Stem Cell 2, 525-528, (2008).
Ordering information
1. C2491-5 (powder) 5mg
2. C2491-5s (10 mM in DMSO)
Product Name: Pyroxamide (C7376)
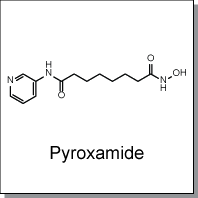
Pyroxamide is a potent histone deacetylase (HDAC) inhibitor with IC50 of 100nM. Pyroxamide induced terminal
differentiation in murine erythroleukemia (MEL) cells, and inhibited the growth by cell cycle arrest or apoptosis in a variety
of tumor cells. Accumulated acetylated histones and increased level of p21/WAF1 expression were detected in cancer
cells and in prostate xenografts treated with pyroxamide.
Details
|
Cocktail Formula : |
|
C13H19N3O3 |
|
CAS # : |
|
382180-17-8 |
|
Molecular Weight : |
|
265.31 |
|
Purity: |
|
>99% |
|
Appearance : |
|
Off-white solid |
|
Storage: |
|
Store solid powder at 4oC desiccated;
Store DMSO solution at -20oC.
|
References
1. Butler et al. Inhibition of transformed cell growth and induction of cellular differentiation by pyroxamide, an inhibitor
of histone deacetylase. Clin.Cancer Res. 7 962. (2001)
2. Kutko, MC., et al. Histone deacetylase inhibitors induce growth suppression and cell death in human
rhabdomyosarcoma in vitro. Clin Cancer Res. Nov 15;9(15):5749-55.(2003)
3. Kouraklis G, Theocharis S. Histone deacetylase inhibitors and anticancer therapy. Curr Med Chem Anticancer Agents.
Jul;2(4):477-84.(2002)
4. Kouraklis G, Theocharis S Histone deacetylase inhibitors: a novel target of anticancer therapy (review). Oncol Rep.
Feb;15(2):489-94. (2006)
Ordering information
1. C7376-5 (powder) 5mg
2. C7376-5s (10 mM in DMSO)
Product Name: Vorinostat (C8670)
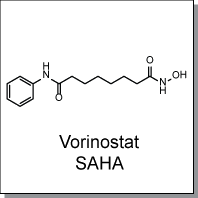
Vorinostat (SAHA) is a potent inhibitor of Classes I and II histone deacetylases (HDACs) that works by chelating Zinc ions
found in the active site of HDACs. Vorinostat's inhibition of HDAC activity results in the accumulation of acetylated
histones and acetylated proteins, including transcription factors crucial for the expression of genes needed to induce
cell differentiation. Vorinostat inhibits the proliferation of both normal cells and a wide variety of transformed cells. It
induces death in tumor cells while leaves normal cells alive. Vorinostat inhibits tumor growth in a variety of animal models.
Marketed under the name Zolinza, Vornostat is a FDA approved medicine for the treatment of cutaneous T cell
lymphoma (CTCL). Vorinostat is now under clinical investigations for several other types of cancers and for its potential
use in eradicating HIV from HIV+ patients.
Details
|
Chemical Formula : |
|
C14H20N2O3 |
|
CAS # : |
|
149647-78-9 |
|
Molecular Weight : |
|
264.32 |
|
Purity: |
|
>99% |
|
Appearance : |
|
White solid |
|
Solubility : |
|
Soluble in DMSO up to 50 mM |
|
Chemical Name : |
|
N-hydroxy-N'-phenyl-octanediamide |
|
Storage: |
|
Store solid powder at 4oC desiccated;
Store DMSO solution at -20oC.
|
References
1. Richon VM. Cancer biology: mechanism of antitumour action of vorinostat (suberoylanilide hydroxamic acid), a novel
histone deacetylase inhibitor. Br J Cancer. 2006 Dec95(S1)
2. Contreras X, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009
284 (11): 6782�9.
3. Archin,NM. et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature.
2012 Jul 25;487(7408):482-5.
Ordering information
1. C8670-10 (powder) 10mg
Product Name: LBH589 (C5245)
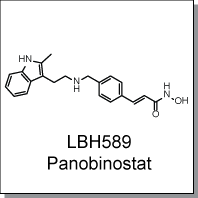
LBH589, a hydroxamate analog, is a broad-spectrum HDAC inhibitor. It has been shown to increase acetylation of core
histones (H3 and H4) and nonhistone proteins (alpha-tublin, HSP90), leading to the modulation of gene expression
(p21, FOXO3A, GADD45A, aromatase, etc) and protein activity involved in cell growth and survival pathways. LBH589
induces apoptosis in MOLT-4 and Reh cells with IC50 between 5 to 20nM. In lung cancer and mesothelioma animal
models, LBH589 markedly decreased tumor growth by 62% when compared with the vehicle control. The anti-tumor
activity of LBH589 has also been demonstrated in many other cancer cell lines, including multiple myeloma, NSCLC and
castrate-resistant prostate cancer cell lines. LBH589 is under clinical trials to evaluate its effects in conjunction with
chemotherapy and/or targeted therapy in multiple cancer types.
Details
|
Chemical Formula : |
|
C21H23N3O2 |
|
CAS # : |
|
404950-80-7 |
|
Molecular Weight : |
|
349.43 |
|
Purity: |
|
>98% |
|
Appearance : |
|
White solid |
|
Solubility : |
|
Soluble in DMSO up to 50 mM |
|
Chemical Name : |
|
(2E)-N-hydroxy-3-[4-({[2-(2-methyl-1H-indol-3-yl)ethyl]amino}methyl)phenyl]
acrylamide |
|
Storage: |
|
Store solid powder at 4oC desiccated;
Store DMSO solution at -20oC.
|
References
1. Scuto, A., et al. The novel histone deacetylase inhibitor, LBH589, induces expression of DNA damage response
genes and apoptosis in Ph- acute lymphoblastic leukemia cells. Blood. 2008 May 15;111(10):5093-100.
2. Chen, S. et al.The HDAC inhibitor LBH589 (panobinostat) is an inhibitory modulator of aromatase gene expression.
Proc Natl Acad Sci U S A 2010 Jun 15; 107(24) :11032-7.
3. George, P., et al. "Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly
active against human CML-BC cells and AML cells with activating mutation of FLT-3." Blood 105: 1768-1776 (2005).
4. Qian, D.Z., et al. "Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative
LBH589." Clin. Cancer Res. 12: 634-642 (2006).
5. Crisanti, MC. et al. The HDAC inhibitor panobinostat (LBH589) inhibits mesothelioma and lung cancer cells in vitro and
in vivo with particular efficacy for small cell lung cancer. Mol Cancer Ther. 2009 Aug;8(8):2221-31.
Ordering information
1. C5245-5 (powder) 5mg
2. C5245-5s (10 mM in DMSO)
- 이전글Repro-PACT™ & other new products of Cellagen Tech. 13.04.24
- 다음글Cellagen Technology LLC's new products 12.12.02
댓글목록
등록된 댓글이 없습니다.