|
Disease Area, Immunology I
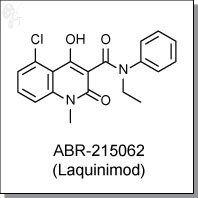
Product Name: ABR-215062 (Laquinimod) l Immunomodulator
 |
|
Laquinimod is an orally-available. quinolinone-based, broad spectrum immunomodulator with anti-
inflammatory activity, most likely by effecting a T-helper cell 1 to 2 cytokine shift. [1] Additionally it reduces
antigen presentation and the effect on migration of T-cells. Laquinimod is also believed to provide a
neuroprotective effect and may have application to relapsing-remitting multiple sclerosis (RRMS).
In animal models for multiple sclerosis, Laquinimod reversed established relapsing remitting experimental
autoimmune encephalomyelitis (RR EAE) and was associated with reduced CNS inflammation, decreased
Th1 and Th17 responses, and an increase in regulatory T cells.
|
Details
|
Chemical Formula:
|
|
C19H17ClN2O3
|
|
CAS No.:
|
|
248281-84-7
|
|
Molecular Weight:
|
|
356.8
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White solid
|
|
Chemical Name:
|
|
5-chloro-N-ethyl-4-hydroxy-1-methyl-2-oxo-N-phenyl-1,2-dihydroquinoline-
carboxamide
|
|
Solubility:
|
|
Up to 22 mM in DMSO
|
|
Synonyms:
|
|
ABR-215062, ABR 215062, ABR215062, Laquinimod
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. "Laquinimod for relapsing-remitting multiple sclerosis" National Horizon Scaning Centre technology
summary, Aug. 2011.
2. West et al., Profile of oral laquinimod and its potential in the treatment of multiple sclerosis. Degenerative
Neurological Muscular Disease 2011, 1, 25-32.
3. Schulze-Topphoff et al., Laquinimod, a quinoline-3-carboxamide, induces type II myeloid cells that
modulate central nervous system autoimmunity. PLoS ONE 2012, 7(3), e33797.
|
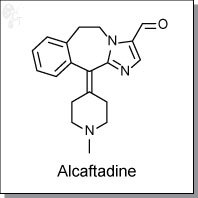
Product Name: Alcaftadine l Histamine (H1/H4) receptor antagonist
 |
|
Alcaftadine, a imidazobenzazepine-based antagonist of the histamine receptors, has a pKi of 8.5 and 5.8 for
the H1 receptor and H4 receptors, respectively. Additionally, Alcaftadine shows higher affinity for the H2
receptor than ketotifen
In support of its potent anti-inflammatory properties, Alcaftadine also exhibits modulatory action on immune
cell recruitment and mast cell stabilizing effects. Receptor binding studies revealed less significant effects
ranging from a moderate affinity for cholinergic muscarinic receptors and lower affinity for human a2A-
adrenoreceptors, serotonin 5-H51A, 5-HT2A receptors, and melanocortin MC4 receptors. [2] Furthermore,
displacement experiments showed that at concentrations up to 10 uM, Alcaftadine did not interact with other
receptors, transporters, or ion channel binding sites, including the hERG channel.
|
Details
|
Chemical Formula:
|
|
C19H21N3O
|
|
CAS No.:
|
|
147084-10-4
|
|
Molecular Weight:
|
|
307.38
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
2-(1-Methylpiperidin-4-ylidene)-4,7-diazatricyclo[8.4.0.0(3,7)]tetradeca- 1(14)
,3,5,10,12-pentaene-6-carbaldehyde
|
|
Solubility:
|
|
Up to 20 mM in DMSO
|
|
Synonyms:
|
|
Alcaftadine, 147084-10-4, Lastacaft
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Gallois-Bernois et al., Alcaftadine, a new antihistamine with combined antagonist activity at histamine H1,
H2, and H4 receptors. J. Receptor Ligand Channel Res. 2012, 5, 9-20.
2. Namdar et al., Alcaftadine: A topical antihistamine for use in allergic conjunctivitis. Drugs of Today, 2011,
47(12), 883-890
3. Bohets et al., Clinical Pharmacology of Alcaftadine, a Novel Antihistamine for the Prevention of Allergic
Conjunctivitis. J. Ocular. Pharm. Ther. 2011, 27(2), 187-195.
|
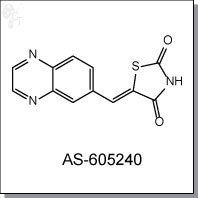
Product Name: AS-605240 l Pl3K inhibitor
 |
|
AS-605420 is an orally-available, ATP competitive inihbitor of the thiazolinedione class with IC50 potency
against PI3Kg of 8 nM. It also has peripheral activity versus other PI3K isoforms of 60 nM, 270 nM, and 300
nM for alpha, beta, and delta, respectively.
AS-605240 has been shown to selectively block class IB PI3K signaling in cultured macrophages. Dose-
dependent treatment resulted in lowered blood glucose levels with improvement in insulin sensitivity and
glucose tolerance without adverse effect on body weight.
AS-605240 has also been utilized in the exploration for experimental autoimmune encephalomyelitis (EAE).
AS605420-treated mice fully recovered from EAE and experienced significantly reduced clinical and
cumulative disease scores. Histological CNS analysis revealed that AS605240 treated mice had reduced
cellular pathology in the spinal cord and reduced signs of demyelination.
|
Details
|
Chemical Formula:
|
|
C12H7N3O2S
|
|
CAS No.:
|
|
648450-29-7
|
|
Molecular Weight:
|
|
257.27
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
Grey
|
|
Chemical Name:
|
|
(Z)-5-(quinoxalin-6-ylmethylene)thiazolidine-2,4-dione
|
|
Solubility:
|
|
Up to 5 mM in DMSO
|
|
Synonyms:
|
|
AS-605240, AS605240, AS605240
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Kobayashi et al., Blockade of class IB phosphoinositide-3 kinase ameliorates obesity-induced
inflammation and insulin resistance. Proc. Natl. Acad. Sci. 2011, 108(14), 5753-5758.
2. Comerford et al., PI3K? drives priming and survival of autoreactive CD4(+) T cells during experimental
autoimmune encephalomyelitis. PLoSONE, 2012, 7(9), e45095
|
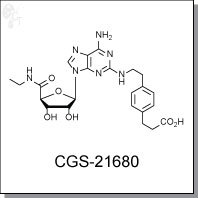
Product Name: GCS-216680 l A2A receptor agonist
 |
|
CGS21680, an A2A adenosine receptor agonist, is under investigation in various inflammation models for
potential novel therapies. For example, CGS21680 possesses an anti-inflammatory effect during chronic
inflammation and lessens the tissue damage associated with collagen-induced arthritis.
CGS21680 reduces spinal cord injury-induced phosphorylation of JNK and MAPK. It was shown also to
reduce the influx of MPO-positive leukocytes, NFkB activation, and iNOS expression. Additionally, CGS21680
reduced phosphorylation of p65 on Ser536.
In acute lung inflammation models, CGS21680 was shown to reduce neutrophil infiltration and thus degree of
lung injury after a carrageenan-challenge.
|
Details
|
Chemical Formula:
|
|
C23H29N7O6
|
|
CAS No.:
|
|
124182-57-6
|
|
Molecular Weight:
|
|
499.52
|
|
Purity:
|
|
> 98%
|
|
Appearance:
|
|
White
|
|
Chemical Name:
|
|
3-[4-[2-[ [6-amino-9-[(2R,3R,4S,5S)-5-(ethylcarbamoyl)-3,4-dihydroxy-oxolan-2-
yl]purin-2-yl]amino]ethyl]phenyl]propanoic acid
|
|
Solubility:
|
|
Up to 100 mM in DMSO
|
|
Synonyms:
|
|
CGS-21680, CGS 21680, CGS21680
|
|
Storage:
|
|
For longer shelf life, store solid powder at 4oC desiccated, or store DMSO solution
at -20oC.
|
|
Reference:
1. Mazzon et al., CGS 21680, an agonist of the adenosine (A2A) receptor, reduces progression of murine
type II collagen-induced arthritis. J. Rheumatol., 2011, 38(10), 2119-2129.
2. Genovese, The selective adenosine A2A receptor agonist CGS 21680 reduces JNK MAPK activation in
oligodendrocytes in injured spinal cord. Shock, 2009, 32(6), 578-585.
3. Impellizzeri et al., CGS 21680, an agonist of the adenosine (A2A) receptor, decreases acute lung
inflammation. Eur. J. Pharmacol. 2011, 668, 305-316
|
|



