GSK126 & other new products of Cellagen Technology
본문
GSK126 & other new products of Cellagentech
Product Name: GSK126
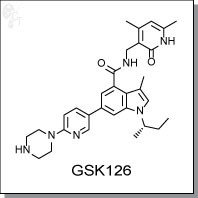
GSK126 is an indole-based inhibitor of EZH2 methyltransferase activity, with an Ki-app of 0.5-3 nM independent of
substrate used. It is competitive with S-adenosyl methionine (SAM) and non-competitive with peptide substrates.
GSK126 is highly selective against many other protein classes and methyltransferases, including both SET-domain-
containing and non-SET-domain-containing classes. GSK126 decreases global H3K27me3 levels and reactivates silenced
PRC2 target genes, as well as inhibits the proliferation of EZH2 mutant DLBCL cell lines and corresopnding xenografts in
mice. The collection of pharmacological data suggests that GSK126 is a potential treatment for EZH2 mutant lymphoma.
[1]
Details
|
Chemical Formula: |
|
C31H38N6O2 |
|
CAS No.: |
|
1346574-57-9 |
|
Molecular weight: |
|
526.67 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
(S)-1-(sec-butyl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-methyl-6-
(6-(piperazin-1-yl)pyridin-3-yl)-1H-indole-4-carboxamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
0 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. McCabe et al., EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature, 2012,
492, 108-114. Pubmed ID: 23051747
Product Name: GNF-2
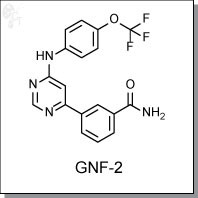
GNF-2 is an aminopyrimidine-based, allosteric inhibitor of Bcr-Abl wih an in vitro IC50 activity of 70 nM. [1] GNF-2 exhibits
preferential activity Bcr-Abl-expressing cells (138 nM) with little to no antiproliferative effects in a panel of Ba/F3 cells
transformed with kinases such as Flt3 ITD, Tel PDGFRb, TPR Met, and Tel JAK1. In Bcr-Abl-transformed cells, GNF-2
induces apoptosis at concentrations as low as 1 uM at 48h, while parental Ba/F3 cells were unaffected at concentrations
up to 10 uM. [2] GNF-2 also inhibits Bcr-Abl and Stat5 tyrosine phosphorylation at concentrations of 270 nM and 1 uM,
respectively. Selectivity panel studies show that GNF-2 is inactive towards a range of serine/threonine (e.g. CDK1, cRAF,
PDK1, PKA, PKB), receptor tyrosine (e.g. FGFR1/3, Flt1/3/4, HER1/2, KDR, c-Kit), and non-receptor tyrosine kinases
(BTK, Lck, c-Src).
GNF-2 targets wild-type Bcr-Abl as well as many clinically-relevant imatinib (Gleevec)-resistant mutants eitehr alone or in
combination with other Bcr-Abl inhibitors. GNF-2 is also known to inhibit Arg (Abl-related gene) kinase at a IC50 of
670 nM. [1]
Details
|
Chemical Formula: |
|
C18H13F3N4O2 |
|
CAS No.: |
|
778270-11-4` |
|
Molecular weight: |
|
374.32 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
Benzamide, 3-[6-[[4-(trifluoromethoxy)phenyl]amino]-4-pyrimidinyl] |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Synonyms: |
|
GNF-2, GNF 2 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Choi et al., N-Myristoylated c-Abl Tyrosine Kinase Localizes to the Endoplasmic Reticulum upon Binding to an Allosteric
Inhibitor. J. Biol. Chem. 2009, 284(42), 29005-29014. Pubmed ID: 19679652
2. Adrian et al., Allosteric inhibitors of Bcr-Abl-dependent cell proliferation. Nat. Chem. Biol. 2006, 2(2), 95-102. Pubmed
ID: 16415863
Product Name: Zanamivir
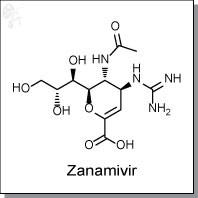
Zanamivir is a dihydropyran-based, sialic acid analog inhibitor of the viral enzyme neuraminidase. Zanamivir's mode of
action is blockage of sialic acid cleavage, thereby preventing further virus distribution. For type A viruses zanamivir has
IC50s of 0.35 nM for the H1N1 subtype, and 1.1 nM for the H3N2 subtype. [1]
In plaque reduction in vitro assays, zanamivir inhibits a wide range of Type A and Type B influenza viruses with IC50s of
plaque formation ranging from 5 to 14 nM in laboratory-passaged strains, and 20 to 1600 nM for clinical isolates. In
human respiratory epithelial cells, EC90s of virus yields were determined to be less than 0.01 ug/mL at 24h for both H1N1
and H3N2 subtypes. [2]
Details
|
Chemical Formula: |
|
C12H20N4O7 |
|
CAS No.: |
|
139110-80-8 |
|
Molecular weight: |
|
332.31 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
(2R,3R,4S)-3-acetamido-4-guanidino-2-((1R,2R)-1,2,3-trihydroxypropyl)-3,4-dihydro-2H-
pyran-6-carboxylic acid |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
Relenza, Zanamavir, Sialic acid derivative |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Colman et al., Zanamivir: an influenza neuraminidase inhibitor. Expert Rev. Anti. Infect. Ther. 2005, 3(2), 191-199.
2. Elliott et al., Zanamivir: from drug design to the clinic. Phil. Trans. R. Soc. Lond. 2001, 356, 1885-1893.
3. McNicholl et al., Neuraminidase Inhibitors: Zanamivir and Oseltamivir. Annals Pharmacother. 2001, 35, 57-70.
Product Name: YM155
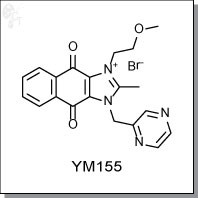
YM155 is an imidazolium-based inhibitor of survivin in a dose-dependent manner when administered from 10 to 1000 nM. YM155 suppresses survivin expression and induces apoptosis in PC-3 and PPC-1 human hormone-refractory prostate
cancer (HRPC) cell lines at 10 nM. In addition to other HRPC cell lines, YM155 also shows activity against malignant
melanoma lines SKMEL5 and A375 at GI50s of 4.2 and 6.3 nM, respectively. [1]
In independent studies, YM155 was shown to inhibit SK-NEP-1 Wilms tumor cells at an IC50 of 100 nM, while
concurrently inducing apoptosis. [2]
YM155 is believed to sensitize NSCLC cells to radiation both in vivo and in vitro by downregulating survivin expression in a
concentration- and time-dependent manner. YM155 delays the repair of radaiation-induced double-strand breaks in
nuclear DNA. [3]
Details
|
Chemical Formula: |
|
C20H19BrN4O3 |
|
CAS No.: |
|
781661-94-7 |
|
Molecular weight: |
|
443.29 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
1H-Naphth[2,3-d]imidazolium, 4,9-dihydro-1-(2-methoxyethyl)-2-methyl-4,9-dioxo-3-
(2-pyrazinylmethyl)-, bromide (1:1) |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Synonyms: |
|
YM155, YM-155 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Nakahara et al., YM155, a Novel Small-Molecule Survivin Suppressant, Induces Regression of Established Human
Hormone-Refractory Prostate Tumor Xenografts. Cancer Res. 2007, 67, 8014-8021. Pubmed ID: 17804712
2. Tao et al., Survivin selective inhibitor YM155 induce apoptosis in SK-NEP-1 Wilms tumor cells. BMC Cancer, 2012, 12,
619-631. Pubmed ID: 23267699
3. Iwasa et al., Radiosensitizing Effect ofYM155, a Novel Small-Molecule Survivin Suppressant, in Non^Small Cell Lung
Cancer Cell Lines. Clin. Cancer Res. 2008, 14, 6496-6504.
Product Name: WYE125132
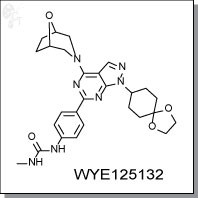
WYE125132 is an orally-available, ATP-competitive, pyrazolopyrimidine-based, specific inhibitor of mTORC1 and mTORC2
with an mTOR potency of 0.19 nM and >5000-fold selectivity against the PI3K isoform family, hSMG1, and ATR enzymes.
WYE125132 selectively inhibits phosphorylation of p-Akt (S473) and Akt without significantly reducing levels of T308.
FACS analysis reveals that WYE125132 induces cell cycle arrest at the G1-phase, with a reduction in S-phase cells. [1]
WYE125132 induces the rapid loss of phosphorylation at Ser75. Maf1 dephosphorylation was also observed and
correlated with its accumulation in the nucleus and a marked decline in the cellular levels of pre-tRNAs. [2]
Details
|
Chemical Formula: |
|
C27H33N7O4 |
|
CAS No.: |
|
1144068-46-1 |
|
Molecular weight: |
|
519.6 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
Urea, N-[4-[1-(1,4-dioxaspiro[4.5]dec-8-yl)-4-(8-oxa-3-azabicyclo[3.2.1]oct-3-yl)-1H-
pyrazolo[3,4-d]pyrimidin-6-yl]phenyl]-N'-methyl |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Synonyms: |
|
WYN125132, WYN-132,WYN-125132 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Yu et al., Beyond Rapalog Therapy: Preclinical Pharmacology and Antitumor Activity of WYE-125132, an ATP-
Competitive and Specific Inhibitor of mTORC1 and mTORC2. Cancer Res. 2010, 70, 621-631. Pubmed ID: 20068177
2. Shor et al., Requirement of the mTOR Kinase for the Regulation of Maf1 Phosphorylation and Control of RNA
Polymerase III-dependent Transcription in Cancer Cells. J. Biol. Chem. 2010, 285(20), 15380-15392.
Pubmed ID: 20233713
Product Name: SYN115 (Tozadenant)
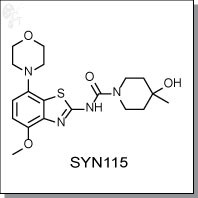
SYN115 (Tozadenant) is a orally-available, benzothiazole-based, antagonist of the Adenosine A2A receptor for the
treatment of Parkinson's disease and other CNS-related conditions. SYN115 is being pursued as a monotherapy as well as
in combination with L-dopamine and is being studied for its potential neuroprotective effects. [1]
Animal studies with SYN115 support beliefs that A2A antagonists reduce Parkinson's symptoms by reducing the inhibitory output of the basal ganglia indirect pathway. Using Cerebral Blood Flow (CBF) as a pharmacodynamic parameter, studies
have shown that SYN115 induces a significant decrease in thalamic CBF, consistent with the deactivation of the indirect
pathway. [2]
Details
|
Chemical Formula: |
|
C19H26N4O4S |
|
CAS No.: |
|
870070-55-6 |
|
Molecular weight: |
|
406.5 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
4-Hydroxy-N-[4-methoxy-7-(4-morpholinyl)-2-benzothiazolyl]-4-methyl-1-
piperidinecarboxamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
SYN115, SYN-115 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Biotie Therapies website (2013-04-24)
2. Black et al., Quantification of Indirect Pathway Inhibition by the Adenosine A2a Antagonist SYN115 in Parkinson
Disease. Black et al., J. Neurosci. 2010, 30(48), 16284-16292. Pubmed ID: 21123574
Product Name: SU5416 (Semaxinib)
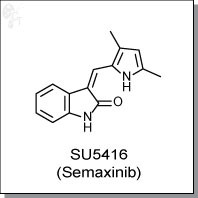
SU5416 (Semaxinib) is a reversible, ATP-competitive, oxindole-based inhibitor of Flk-1/KDR receptor tyrosine kinase.
SU5416 inhibits VEGF-dependent phosphorylation of the Flk-1 receptor in Flk-1-overexpressing NIH 3T3 cells with a IC50 of
1.04 uM. In an ELISA-based assay, SU5416 inhibits autophosphorylation of the Flk-1 receptor at an IC50 of 1.23 uM. [1]
In a mitogenic/proliferation assay in HUVECs, the IC50 of SU5416 is extremely time-dependent, ranging from 1 uM to
40 nM at 48h incubation. These data in conjunction with dose-ranging studies support regimens of less than once-daily
dosing of SU5416. [2]
More recent studies have shown that SU5416 is also an agonist of the aryl hydrocarbon receptor (AHR), leading to the
generation of regulatory T-cells in vitro. SU5416 also upregulates CYP1A1 and CYP1B1. [3]
Details
|
Chemical Formula: |
|
C15H14N2O |
|
CAS No.: |
|
204005-46-9 |
|
Molecular weight: |
|
238.28 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
2H-Indol-2-one, 3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-1,3-dihydro-, (3Z) |
|
Solubility: |
|
Up to 20 mM in DMSO |
|
Synonyms: |
|
SU5416, SU 5416, su-5416, Semaxinib |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Fong et al., SU5416 Is a Potent and Selective Inhibitor of the Vascular Endothelial Growth Factor Receptor
(Flk-1/KDR) That Inhibits Tyrosine Kinase Catalysis, Tumor Vascularization, and Growth of Multiple Tumor Types. Cancer
Res. 1999, 59, 99-106. Pubmed ID: 9892193
2. Mendel et al., The Angiogenesis Inhibitor SU5416 Has Long-lasting Effects on Vascular Endothelial Growth Factor
Receptor Phosphorylation and Function. Clin. Cancer Res. 2000, 6, 4848-4858. Pubmed ID: 11156244
3. Mezrich et al., SU5416, a VEGF Receptor Inhibitor and Ligand of the AHR, Represents a New Alternative for
Immunomodulation. PLOS One, 2012, 7(9), e44547.
Product Name: Prucalopride
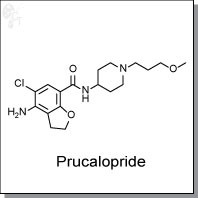
Prucalopride is an orally-available, dibenzofuran-based, enterokinetic agonist of the human serotonin 5-HT4a and 5-HT4b
receptor isoforms with Ki estimates of 3 nM and 8 nM, respectively. It has modest selectivity over the human D4
receptor (2.3 uM), mouse 5-HT3 receptor (3.7 uM) and human s1 receptor (3.7 uM). (1, 2)
Initial tolerability studies show that prucalopride does not encounter cardiotoxicity issues to the extent seen in other
drugs of this class. [3]
Details
|
Chemical Formula: |
|
C18H26CIN3O3 |
|
CAS No.: |
|
179474-81-8 |
|
Molecular weight: |
|
367.87 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
7-Benzofurancarboxamide, 4-amino-5-chloro-2,3-dihydro-N-[1-(3-methoxypropyl)-4-
piperidinyl] |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Synonyms: |
|
0 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Briejer et al., The in vitro pharmacological profile of prucalopride, a novel enterokinetic compound, Eur. J. Pharmcol.
2001, 423, 71-83. Pubmed ID: 11438309
2. Frampton et al., Prucalopride: ADIS Drug Profile. Drugs, 2009, 69(17), 2453-2476. Pubmed ID: 19911858
3. Quigley et al., Prucalopride: safety, efficacy and potential applications. Ther. Adv. Gastroenterol. 2012, 5(1), 23-30.
Ordering information
|
Catalog No. |
Product Name |
Size |
|
C4126-2s |
GSK126 |
2mg, 10mg & 50mg |
|
C4632-5 |
GNF-2 |
5mg, 25mg & 100mg |
|
C9262-2s |
Zanamivir |
2mg, 10mg & 50mg |
|
C9615-5 |
YM155 |
5mg, 25mg & 100mg |
|
C9125-2s |
WYE125132 |
2mg, 10mg & 50mg |
|
C7911-5 |
SYN115 (Tozadenant) |
5mg, 25mg & 100mg |
|
C7541-5 |
SU5416 (Semaxinib) |
5mg, 25mg & 100mg |
|
C7782-5 |
Prucalopride |
5mg, 25mg & 100mg |
▣ 관련 페이지 ; Cellangen Technology
- 이전글VX-765 & other new products of Cellagen Technology 13.07.08
- 다음글Repro-PACT™ & other new products of Cellagen Tech. 13.04.24
댓글목록
등록된 댓글이 없습니다.