Recombinant selenoproteins, Human Thioredoxin Reductase
본문
|
Recombinant selenoproteins, Human Thioredoxin Reductase
SeLENOZYME uses a cutting-edge technology, resulting from over 30 years of research at Karolinska Institutet in Stockholm, Sweden. Their recombinant system allows them to produce purified and active selenoproteins from human and most other naturally occurring selenoproteins, or other tailored synthetic selenocysteine-containing proteins. At present SeLENOZYME has more than 25 selenoproteins in their portfolio.After request SeLENOZYME has the ability to produce all 837 selenoproteins yet identified in nature, as well as synthetic selenoproteins tailored to customer requirements.
SeLENOZYME's recombinant production system uses bacteria instead of mammalian cells, making the production of active selenoproteins more effective and cost-efficient. Selenozyme has established a state-of- the-art protein production facility to supply our customers with selenoproteins at any desired scale and purity and at very competitive prices compared to selenoproteins purified from native sources.
All selenoproteins contain a rare amino acid, selenocysteine, which has limited their use in research, therapy and diagnostics. Up until now, customers have been forced to purchase either inactive selenoproteins, lacking the essential selenocysteine residue, or active selenoproteins obtained from animal sources, being expensive, ethically questionable and providing very low yields.
Since all selenoproteins by definition contain the rare amino acid Selenocysteine (Sec), it has previously been very hard to obtain them in pure form or to produce them recombinantly, due to the complex genetic system required for co-translational Sec insertion.
Recombinant Human Thioredoxin Reductase 1 Non-tagged, 499 residues
The human TrxR1 selenoprotein is encoded by the TXNRD1 gene and exerts a wide range of functions in cytosolic redox control and antioxidant systems. SeLENOZYME provide the main cytosolic isoform of the human TrxR1 enzyme with more than 90% Sec contents and more than 95% purity in aliquots at amounts from 5 units to 50 units. Other specific isoforms of TrxR1 can be made to order.
Recombinant Human Thioredoxin Reductase 2 Non-tagged, No signal peptide, 488 residues
The human TrxR2 selenoprotein is encoded by the TXNRD2 gene and exerts a wide range of functions in mitochondrial redox control and antioxidant systems. SeLENOZYME provide the main mitochondrial isoform of the human TrxR2 enzyme with more than 90% Sec contents and more than 95% purity in aliquots at amounts from 5 units to 50 units. Other specific isoforms of TrxR2 can be made to order.
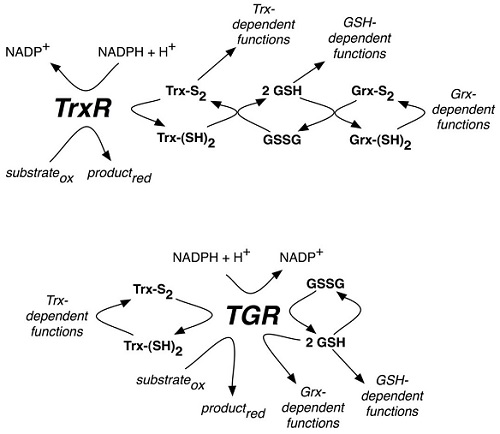
Technology Scheme of reactions catalyzed by mammalian TrxR or parasite TGR selenoproteins From Arnér ES. Focus on mammalian thioredoxin reductases–important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790:495-526.
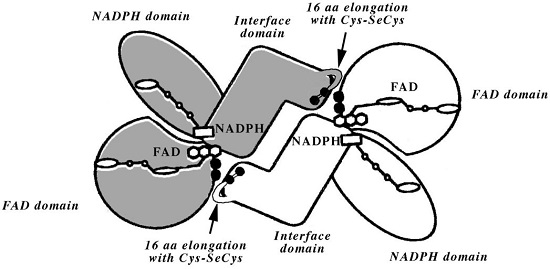
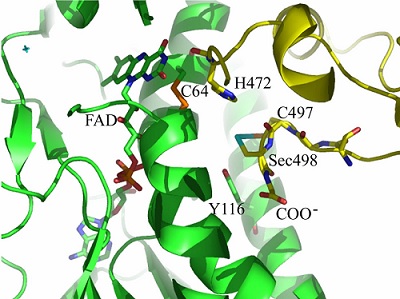
Model of the selenoenzyme TrxR architecture, with its C-terminal arm containing Sec From Zhong L, Arnér ES, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine- selenocysteine sequence. Proc Natl Acad Sci U S A. 2000;97:5854-5
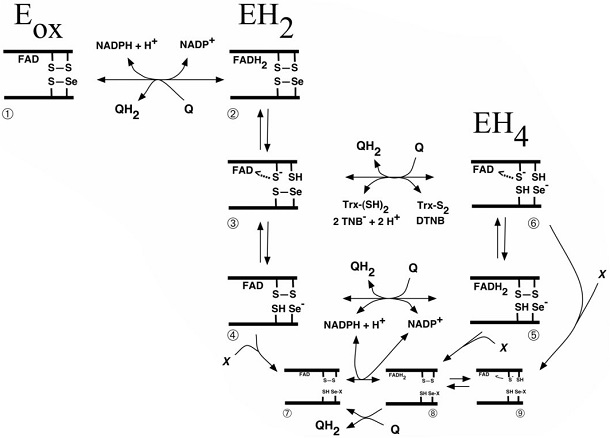
Scheme of quinone interactions with the Sec-containing active site of TrxR From Cenas N, Nivinskas H, Anusevicius Z, Sarlauskas J, Lederer F, Arnér ES. Interactions of quinones with thioredoxin reductase: a challenge to the antioxidant role of the mammalian selenoprotein. J Biol Chem. 2004;279:2583-2592.
Model of the C-terminal Sec-containing site of TrxR1 as based on the crystal structure From Cheng Q, Sandalova T, Lindqvist Y, Arnér ES. Crystal structure and catalysis of the selenoprotein thioredoxin reductase 1. J Biol Chem. 2009;284:3998-4008
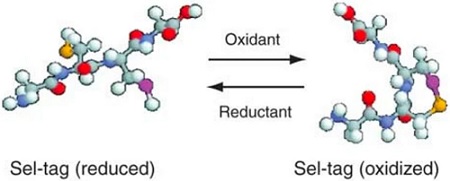
Scheme of a Sel-tag in reduced selenolthiol as well as oxidized selenenylsulfide forms From Johansson, L., Chen, C., Thorell, J. et al. Exploiting the 21st amino acid—purifying and labeling proteins by selenolate targeting. Nat Methods 1, 61–66 (2004)
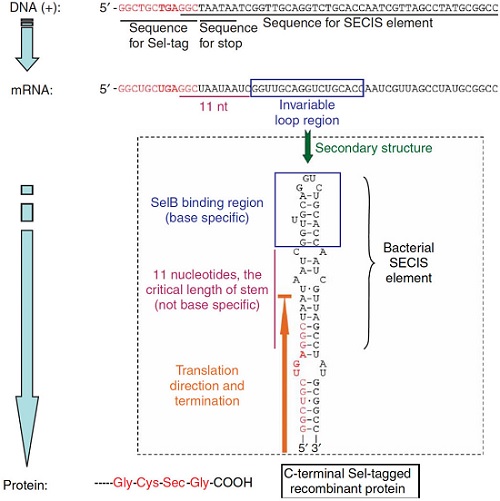
Principle of making Sel-tagged proteins From Cheng, Q., Stone-Elander, S. & Arnér, E. Tagging recombinant proteins with a Sel-tag for purification, labeling with electrophilic compounds or radiolabeling with 11C. Nat Protoc 1, 604–613 (2006)
|
Ordering informations
|
Catalog No. |
Product Name |
Size |
|
HTRXR1 (PDF) |
Recombinant Human Thioredoxin Reductase 1 |
5 / 10/ 20/ 50 units |
|
HTRXR2 (PDF) |
Recombinant Human Thioredoxin Reductase 2 |
▣ 관련 페이지 ; SeLENOZYME
댓글목록
등록된 댓글이 없습니다.