VX-765 & other new products of Cellagen Technology
본문
VX-765 & other new products of Cellagen Technology
Product Name: VX-765
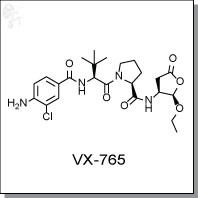
VX-765 is an orally-available prodrug of VRT-043198, an inhibitor of interleukin-converting enzyme / caspase-1 subfamily
caspases. VRT-043198 shows Ki values of 0.8 nM and 100-fold selectivity over other non-ICE subfamily caspases.
VRT-043198 showed no significant activity towards trypsin or cathepsin B. (1)
VRT-043198 inhibits IL-1b release from both PBMCs and whole blood with IC50 values of 0.67 and 1.9 uM, respectively.
Additionally in stimulated PBMCs, VRT-043198 dose-dependently inhibited IL-1b, IL-18, and IFN-g, without affecting TNF-a
release. In a hypoxia-induced apoptosis assay employing the NT2 human neuroblastoma cell line, VRT-043198 did not
alter ischemia-induced apoptosis up to concentrations of 100 uM. (1)
According to Vertex's news release , VX-765 has been shown to inhibit acute seizures in preclinical models of acute
epilepsy and has shown activity in preclinical models of chronic epilepsy that do not respond to standard anti-epileptic
drugs. (2)
Details
|
Chemical Formula: |
|
C24H33CIN4O6 |
|
CAS No.: |
|
273404-37-8 |
|
Molecular weight: |
|
509 |
|
Purity: |
|
> 99% |
|
Appearance: |
|
White |
|
Chemical name: |
|
(S)-1-(sec-butyl)-N-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-3-methyl-
6-(6-(piperazin-1-yl)pyridin-3-yl)-1H-indole-4-carboxamide |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
VX-765, VX-765 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Wannamaker et al., (S)-1-((S)-2-{[1-(4-Amino-3-chloro-phenyl)-methanoyl]-amino}-3,3-dimethyl-butanoyl)-pyrrolidine-
2-carboxylic acid ((2R,3S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide (VX-765), an Orally Available Selective
Interleukin (IL)-Converting Enzyme/ Caspase-1 Inhibitor, Exhibits Potent Anti-Inflammatory Activities by Inhibiting the
Product Name: Tolrestat
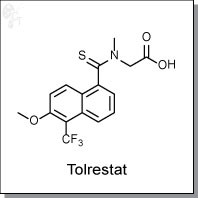
Tolrestat is a thioamide-based inhibitor of aldose reductase with an IC50 activity of 35 nM. (1) Along with other similar
therapies, Tolrestat has implications in the treatment of diabetic complications, such as diabetic neuropathy, in a manner
independent of insulin-related control of blood glucose levels. (2)
In rat diabetes models, Tolrestat reduces sorbitol concentrations in the sciatic nerve and eye lens, as well as prevents
diabetic retinopathy, cateracts, thickening of the retinal basement membrane, and nerve dysfunction. Additionally,
Tolrestat reduces urinary albumin excretion. (3)
Details
|
Chemical Formula: |
|
C16H14F3NO3S |
|
CAS No.: |
|
82964-04-3` |
|
Molecular weight: |
|
357.35 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
2-(6-methoxy-N-methyl-5-(trifluoromethyl)naphthalene-1-carbothioamido)acetic acid |
|
Solubility: |
|
Up to 22 mM in DMSO |
|
Synonyms: |
|
Tolrestat |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Sestanj et al., N-[[5 -(Trifluoromethyl)-6-methoxy-1-naphthalenyl]thioxomethyl]-N-methylglycine (Tolrestat), a Potent,
2. Kador et al., Aldose Reductase Inhibitors: A Potential New Class of Agents for the Pharmacological Control of Certain
Diabetic Complications. J. Med. Chem. 1985, 28(7), 841-849. Pubmed ID: 3925146
3. Fruncillo et al., Pharmacokinetics of the aldose reductase inhibitor tolrestat: Studies in healthy young and elderly male
and female subjects and in subjects with diabetes. Clin. Pharmcol. Ther. 1996, 59(6), 602-613. Pubmed ID: 8681485
Product Name: TMC207 (Bedaquiline)
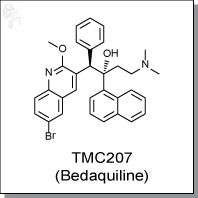
TMC207 (Bedaquiline) is a diarylquinoline-based inhibitor of bacterial ATP synthase at nanomolar concentrations. In vitro
mycobacterial experiments show that TMC207 inhibits drug-sensitive and drug-resistant mycobacterial TB at an MIC range of 2 to 60 nM and an MIC50 of 30 ng/mL. Additionally TMC207 is broadly potent against many non-tuberculous
mycobacteria, with MICs of 6 to 500 nM. (1)
Studies show that TMC207 is orally-available and efficacious on a once-daily administration for patients with pulmonary
TB. No serious adverse events were observed. (2)
Details
|
Chemical Formula: |
|
C32H31BrN2O2 |
|
CAS No.: |
|
843663-66-1 |
|
Molecular weight: |
|
555.5 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
(1R,2S)-1-(6-bromo-2-methoxyquinolin-3-yl)-4-(dimethylamino)-2-(naphthalen-1-yl)-1-
phenylbutan-2-ol |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
TMC207, TMC-207, Bedaquiline |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
Reference
1. Matteelli et al., TMC207: the first compoudn of a new class of potent anti-tuberculosis drugs. Future Microbiol.
2010, 5(6), 849-858. Pubmed ID: 20521931
2. Rustomjee et al., Early Bactericidal Activity and Pharmacokinetics of the Diarylquinoline TMC207 in Treatment of
Product Name: TMC125(Etravirine)
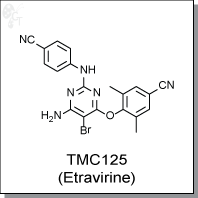
TMC125 is a nonnucleoside reverse transcriptase inhibitor with an EC50 of 1.4 to 4.3 nM for wild-type HIV-1. Additionally,
TMC125 is highly potent against a wide range of single-mutant and double-mutant NNRTI-resistant HIV-1 strains,
including L100I, K103N, Y181C, and L100I+K103N, at EC50s of 3, 1, 7, and 19 nM, respectively. (1)
TMC125 is believed to have a high barrier to development of resistance in vitro based on multiplicity of infection
experiments which confirm a profile distinct from other reverse transcriptase inhibitors. (2)
Details
|
Chemical Formula: |
|
C20H15BrN6O |
|
CAS No.: |
|
269055-15-4 |
|
Molecular weight: |
|
435.28 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
4-((6-amino-5-bromo-2-((4-cyanophenyl)amino)pyrimidin-4-yl)oxy)-3,5-
dimethylbenzonitrile |
|
Solubility: |
|
Up to 50 mM in DMSO |
|
Synonyms: |
|
TMC125, TMC-125, Etravirine |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References:
1. Andries et al., TMC125, a Novel Next-Generation Nonnucleoside Reverse Transcriptase Inhibitor Active against
Nonnucleoside Reverse Transcriptase Inhibitor-Resistant Human Immunodeficiency Virus Type 1. Antimicrob. Agents
2. Vingerhoets et al., TMC125 Displays a High Genetic Barrier to the Development of Resistance: Evidence from In Vitro
Product Name: RAD001 (Everolimus)
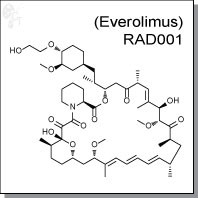
RAD001 (Everolimus) is an orally-bioavailable, semi-synthetic compound with immunosuppressive activity. It has an IC50
against FKBP12 (FK506-binding protein 12) of 1.8-2.6 nM. Moreover, in an IL-6-dependent hybridoma clone, growth
factor-stimulated cell proliferation was measured at an IC50 of 0.2-1.4 nM. Immunosuppressant activity was measured
in a mouse lymphocyte reaction model and was determined to be 0.2-1.6 nM. In a Human T-cell clone model, its IC50
was 0.05-0.17 nM. (1)
Everolimus binds to FKBP12, thus forming a complex that inhibits mTOR activity and concomitantly reduces downstream
markers such as S6 ribosomal protein kinase (S6K1) and eukaryotic elongation factor 4E-binding protein (4EBP). (2)
Everolimus has been used in combination with agents such as Letrozole to inhibit proliferation and trigger apoptosis,
which has implications in therapies for hormone-dependent breast cancers. (3)
Details
|
Chemical Formula: |
|
C53H83NO14 |
|
CAS No.: |
|
159351-69-6 |
|
Molecular weight: |
|
958.22 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
White |
|
Chemical name: |
|
40-O-(2-hydroxyethyl) derivative of sirolimus |
|
Solubility: |
|
Up to 30 mM in DMSO |
|
Synonyms: |
|
RAD-001, RAD001, Everolimus |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References
1. Nashan, B. et al., Review of the proliferation inhibitor everolimus. Expert Opin. Investig. Drugs. 2002, 11(12),
1845-1857. Pubmed ID: 12457444
2. Atkins et al., Everolimus. Nat. Rev. Drug Disc. 2009, 8, 535-536. Pubmed ID: 19568281
3. Boulay et al., Dual Inhibition of mTOR and Estrogen Receptor Signaling In vitro Induces Cell Death inModels of Breast
Product Name: PSI-6206 (RO2433)
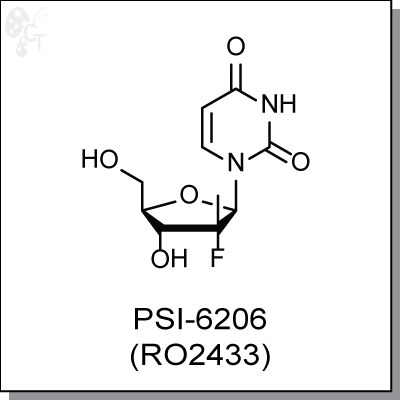
PSI-6206 (RO2433) is the unphosphorylated parent compound of triphosphate analog PSI-7409, which is a potent
inhibitor of the HCV NS5B RNA dependent RNA polymerase. The monophosphate form of PSI-6206 was shown to be
metabolized in primary human hepatocytes to its triphosphate analog PSI-7409. Furthermore, the phosphoramidate
prodrug of PSI-6206 monophosphate, PSI-7851, was developed. Alternatively, PSI-6130, an aminated analog of
PSI-6206 monophosphate, was also developed. (1,2,3)
PSI-7409, the triphosphate of PSI-6206 inhibits wild-type and S282T HCV RdRp with Ki values of 0.42 and 22 uM,
respectively. PSI-7851, the phosphoramidate of PSI-6206 monophosphate, showed an EC50 value of 1.62 uM for
inhibiting HCV RNA replication. (2)
Details
|
Chemical Formula: |
|
C10H13FN2O5 |
|
CAS No.: |
|
863329-66-2 |
|
Molecular weight: |
|
260.22 |
|
Purity: |
|
> 98% |
|
Appearance: |
|
Clear Crystal |
|
Chemical name: |
|
(2'R)-2'-Deoxy-2'-fluoro-2'-methyluridine |
|
Solubility: |
|
Up to 100 mM in DMSO |
|
Synonyms: |
|
PSI-6206, RO-2433, PSI6206, RO2433 |
|
Storage: |
|
Store solid powder at 4oC, or store DMSO solution at -20oC |
References
1. Rodriguez-Torres et al., Antiviral Activity, Pharmacokinetics, Safety, and Tolerability of PSI-7851, a Novel Nucleotide
Polymerase Inhibitor for HCV, Following Single and 3 Day Multiple Ascending Oral Doses in Healthy Volunteers and
Patients with Chronic HCV Infection. 60th AASLD Annual Meeting, 2009.
2. Murakami et al., The Mechanism of Action of b-D-2'-Deoxy-2'-Fluoro-2'-C-Methylcytidine Involves a Second Metabolic
Pathway Leading to b-D-2'-Deoxy-2'-Fluoro-2'-C-Methyluridine 5'-Triphosphate, a Potent Inhibitor of the Hepatitis C
Virus RNA-Dependent RNA Polymerase. Antimicrob. Agents. Chemother. 2008, 52(2), 458-464.
Pubmed ID: 17999967
3. Ma et al., Characterization of the Metabolic Activation of Hepatitis C Virus Nucleoside Inhibitor b-D-2'-Deoxy-2'-fluoro-2'-
C-methylcytidine (PSI-6130) and Identification of a Novel Active 5'-Triphosphate Species. J. Biol. Chem.
2007, 282, 29812-29820. Pubmed ID: 17698842
Ordering information
|
Catalog No. |
Product Name |
Size |
|
C8976-5 |
VX-765 |
5mg, 25mg & 100mg |
|
C8657-5 |
Tolrestat |
5mg, 25mg & 100mg |
|
C8207-5 |
TMC207 (Bedaquiline) |
5mg, 25mg & 100mg |
|
C8125-5 |
TMC125 (Etravirine) |
5mg, 25mg & 100mg |
|
C7230-5 |
RAD001 (Everolimus) |
5mg, 25mg & 100mg |
|
C7620-2 |
PSI-6206 (RO2433) |
2mg, 10mg & 50mg |
- 이전글Current top sellers & new products of CellagenTech 13.07.19
- 다음글GSK126 & other new products of Cellagen Technology 13.05.20
댓글목록
등록된 댓글이 없습니다.